Eczema Clinical Trial Pipeline Accelerates as 100+ Pharma Companies Rigorously Develop Drugs for Market Entry | DelveInsight
Eczema, a chronic inflammatory skin condition, significantly impacts patients’ quality of life through persistent itching and irritation. The market is being driven by growing awareness, increasing diagnosis rates, and the expanding availability of advanced therapies. The introduction of biologics and targeted treatments has redefined disease management by addressing immune mechanisms more precisely. These innovations are fueling strong market momentum and shaping a more dynamic therapeutic landscape.
New York, USA, Dec. 08, 2025 (GLOBE NEWSWIRE) -- Eczema Clinical Trial Pipeline Accelerates as 100+ Pharma Companies Rigorously Develop Drugs for Market Entry | DelveInsight
Eczema, a chronic inflammatory skin condition, significantly impacts patients’ quality of life through persistent itching and irritation. The market is being driven by growing awareness, increasing diagnosis rates, and the expanding availability of advanced therapies. The introduction of biologics and targeted treatments has redefined disease management by addressing immune mechanisms more precisely. These innovations are fueling strong market momentum and shaping a more dynamic therapeutic landscape.
DelveInsight’s 'Eczema Pipeline Insight 2025' report provides comprehensive global coverage of pipeline therapies for eczema across various stages of clinical development. The report offers an in-depth analysis of key trends, emerging therapies, and competitive landscape dynamics, highlighting the strategies of major pharmaceutical companies to advance the pipeline and capitalize on future growth opportunities. In addition, it includes critical insights into clinical trial benchmarking, partnering and licensing activities, and regulatory pathways involving the FDA and EMA, enabling stakeholders to make informed decisions and optimize development strategies within the eczema domain.
Eczema Clinical Trial Analysis Summary
- DelveInsight’s eczema pipeline report depicts a robust space with 100+ active players working to develop 100+ pipeline eczema drugs.
- Key eczema companies such as Sanofi, LEO Pharma, UCB Biopharma, Concerto Biosciences, Corvus Pharmaceuticals, Sitryx Therapeutics, Kymera Therapeutics, Inc., Celldex Therapeutics, Inc., Nektar Therapeutics, Apogee Therapeutics, Inc., E-nitiate Biopharmaceuticals (Hangzhou) Co., Ltd, Enveda, Johnson & Johnson, Hoffmann-La Roche, Evommune, Inc., Artax Biopharma, Attovia Therapeutics Inc, Genrix (Shanghai) Biopharmaceutical Co., Ltd., Pfizer, Jiangsu Simcere Pharmaceutical Co., Ltd., and others are evaluating new eczema drugs to improve the treatment landscape.
- Promising pipeline eczema therapies, such as Amlitelimab, LP 0145, UCB9741, ENS-002, Soquelitinib, SYX-5219, KT-621, Barzolvolimab, Rezpegaldesleukin, APG777, QY201, ENV-294, JNJ-5939, Afimkibart, EVO756, AX-158, ATTO-3712, GR2002, PF-08049820, SIM0278, and others, are in different phases of eczema clinical trials.
- Approximately 10+ eczema drugs are in the late stage of development, whereas 50+ drugs are in the mid and early stages of development.
- Notable MoAs in eczema clinical trials include OX40 ligand inhibitors, Interleukin 22 receptor antagonists, Immunomodulators, ITK (interleukin-2-inducible T cell kinase) Inhibitors, PKM2 modulator, STAT6 transcription factor degraders, KIT Antagonist, Interleukin 13 inhibitors, Janus kinase 1 inhibitors; TYK2 kinase inhibitors, MRGPRX2 protein inhibitors, IL31 protein inhibitors; Interleukin 13 inhibitors, and others.
Request a sample and discover the recent advances in eczema drugs @ Eczema Pipeline Report
What is Eczema?
Eczema, also known as atopic dermatitis, is the most common type of dermatitis. Both genetic and environmental factors influence its development. While eczema is most frequently observed in children, it can also affect adults. Individuals with eczema typically experience dry, itchy skin that is susceptible to infections. The condition is often referred to as the “itch that rashes” because scratching or rubbing the dry skin can trigger a rash. The cornerstone of eczema management is maintaining skin hydration, with topical steroids used to control flare-ups. The lifetime prevalence of atopic dermatitis is estimated at 15–30% in children and 2–10% in adults, with around 60% of cases appearing within the first year of life. Eczema occurs more often in rural than urban settings, highlighting the role of lifestyle and environmental factors in its development.
Find out more about eczema drugs @ Eczema Treatment
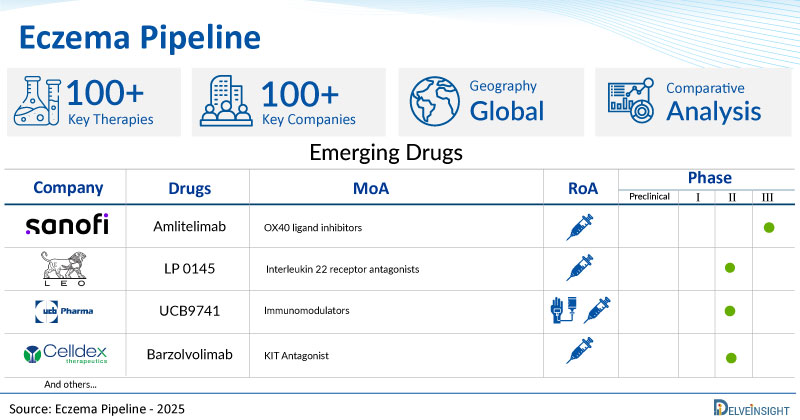
A snapshot of the Pipeline Eczema Drugs mentioned in the report:
| Drugs | Company | Phase | MoA | RoA |
| Amlitelimab | Sanofi | III | OX40 ligand inhibitors | Subcutaneous |
| LP 0145 | LEO Pharma | II | Interleukin 22 receptor antagonists | Subcutaneous |
| UCB9741 | UCB Biopharma | II | Immunomodulators | Intravenous/Subcutaneous |
| Barzolvolimab | Celldex Therapeutics, Inc. | II | KIT Antagonist | Subcutaneous |
| Soquelitinib | Corvus Pharmaceuticals | I | ITK (interleukin-2-inducible T cell kinase) Inhibitors | Oral |
| SYX-5219 | Sitryx Therapeutics | I | PKM2 modulator | Oral |
| ATTO-3712 | Attovia Therapeutics Inc | I | IL31 protein inhibitors; Interleukin 13 inhibitors | Intravenous |
Learn more about the emerging eczema therapies @ Eczema Clinical Trials
Recent Developments in Eczema Treatment Space
- In October 2025, Concerto Biosciences announced positive topline results from its first-in-human Phase Ib clinical trial evaluating ENS-002, a topical three-strain live biotherapeutic product for mild-to-moderate atopic dermatitis (AD).
- In October 2025, Sitryx Therapeutics announced it had received clearance from the US Food and Drug Administration (FDA) for its Investigational New Drug (IND) application for SYX-5219, to support the initiation of a Phase Ib trial in adults with moderate to severe atopic dermatitis in the United States.
- In September 2025, Sanofi announced positive results from the global COAST 1 Phase III study. Amlitelimab met all primary and key secondary endpoints, demonstrating statistically significant and clinically meaningful skin clearance and disease severity compared to placebo at Week 24 in patients aged 12 years and older with moderate-to-severe atopic dermatitis (AD).
- In September 2025, UCB announced new 12-week efficacy and 18-week safety data from the Phase I/IIa first-in-patient trial for Galvokimig, currently under clinical investigation for adults living with moderate-to-severe atopic dermatitis (AD).
- In June 2025, Corvus Pharmaceuticals, Inc. announced new interim data from the randomized, double-blind, placebo-controlled Phase I clinical trial evaluating soquelitinib in patients with moderate to severe atopic dermatitis.
- In May 2025, Enveda announced the successful completion of its Phase I clinical trial of ENV-294, a novel oral therapeutic for atopic dermatitis.
- In May 2025, LEO Pharma A/S announced positive topline results of the Phase IIb trial with temtokibart for the potential treatment of adults with moderate-to-severe atopic dermatitis (AD).
- In April 2025, Kymera Therapeutics, Inc. announced that it had recently initiated dosing in its BroADen Phase Ib clinical trial evaluating KT-621, an oral, highly selective, potent degrader of STAT6, in patients with moderate to severe atopic dermatitis (AD).
- In December 2024, Celldex Therapeutics, Inc. announced that the company had initiated a Phase II study of barzolvolimab in atopic dermatitis (AD) and that the study is actively enrolling patients.
- In May 2024, Apogee Therapeutics, Inc. announced that it had initiated dosing in the Phase II trial of APG777 in patients with moderate-to-severe atopic dermatitis.
Scope of the Eczema Pipeline Report
- Coverage: Global
- Therapeutic Assessment By Product Type: Mono, Combination, Mono/Combination
- Therapeutic Assessment By Clinical Stages: Discovery, Pre-clinical, Phase I, Phase II, Phase III
- Therapeutics Assessment By Route of Administration: Intra-articular, Intraocular, Intrathecal, Intravenous, Ophthalmic, Oral, Parenteral, Subcutaneous, Topical, Transdermal
- Therapeutics Assessment By Molecule Type: Oligonucleotide, Peptide, Small molecule
- Therapeutics Assessment By Mechanism of Action: OX40 ligand inhibitors, Interleukin 22 receptor antagonists, Immunomodulators, ITK (interleukin-2-inducible T cell kinase) Inhibitors, PKM2 modulator, STAT6 transcription factor degraders, KIT Antagonist, Interleukin 13 inhibitors, Janus kinase 1 inhibitors; TYK2 kinase inhibitors, MRGPRX2 protein inhibitors, IL31 protein inhibitors; Interleukin 13 inhibitors
- Key Eczema Companies: Sanofi, LEO Pharma, UCB Biopharma, Concerto Biosciences, Corvus Pharmaceuticals, Sitryx Therapeutics, Kymera Therapeutics, Inc., Celldex Therapeutics, Inc., Nektar Therapeutics, Apogee Therapeutics, Inc., E-nitiate Biopharmaceuticals (Hangzhou) Co.,Ltd, Enveda, Johnson & Johnson, Hoffmann-La Roche, Evommune, Inc., Artax Biopharma, Attovia Therapeutics Inc, Genrix (Shanghai) Biopharmaceutical Co., Ltd., Pfizer, Jiangsu Simcere Pharmaceutical Co., Ltd., and others.
- Key Eczema Pipeline Therapies: Amlitelimab, LP 0145, UCB9741, ENS-002, Soquelitinib, SYX-5219, KT-621, Barzolvolimab, Rezpegaldesleukin, APG777, QY201, ENV-294, JNJ-5939, Afimkibart, EVO756, AX-158, ATTO-3712, GR2002, PF-08049820, SIM0278, and others.
Dive deep into rich insights for new eczema treatments, visit @ Eczema Drugs
Table of Contents
| 1. | Eczema Pipeline Report Introduction |
| 2. | Eczema Pipeline Report Executive Summary |
| 3. | Eczema Pipeline: Overview |
| 4. | Analytical Perspective In-depth Commercial Assessment |
| 5. | Eczema Clinical Trial Therapeutics |
| 6. | Eczema Pipeline: Late-Stage Products (Pre-registration) |
| 7. | Eczema Pipeline: Late-Stage Products (Phase III) |
| 8. | Eczema Pipeline: Mid-Stage Products (Phase II) |
| 9. | Eczema Pipeline: Early-Stage Products (Phase I) |
| 10. | Eczema Pipeline Therapeutics Assessment |
| 11. | Inactive Products in the Eczema Pipeline |
| 12. | Company-University Collaborations (Licensing/Partnering) Analysis |
| 13. | Key Companies |
| 14. | Key Products in the Eczema Pipeline |
| 15. | Unmet Needs |
| 16. | Market Drivers and Barriers |
| 17. | Future Perspectives and Conclusion |
| 18. | Analyst Views |
| 19. | Appendix |
For further information on the eczema pipeline therapeutics, reach out @ Eczema Therapeutics
Related Reports
Eczema Epidemiology Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted eczema epidemiology in the 7MM, i.e., the United States, EU5 (Germany, Spain, Italy, France, and the United Kingdom), and Japan.
Chronic Hand Eczema Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key chronic hand eczema companies, including LEO Pharma, Japan Tobacco, Asana Biosciences, among others.
Chronic Hand Eczema Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key chronic hand eczema companies, including LEO Pharma, Japan Tobacco, Asana Biosciences, among others.
Atopic Dermatitis Market Insights, Epidemiology, and Market Forecast – 2034 report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key atopic dermatitis companies including Arcutis Biotherapeutics, Amgen, Kyowa Kirin, Dermavant Sciences, Cara Therapeutics, Pfizer, Arena Pharmaceuticals, BioMimetix, Eli Lilly and Company, Aldeyra Therapeutics, Inc., Hangzhou Yirui Pharmaceutical Technology Co., Ltd, LEO Pharma, Corvus Pharmaceuticals, Inc., Brexogen Inc., Sanofi, Shaperon, UCB Pharma, Q32 Bio Inc., Akeso, Apogee Therapeutics, Inc., Allakos Inc., Biosion, Inc., among others.
Atopic Dermatitis Clinical Trial Analysis
Atopic Dermatitis Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key atopic dermatitis companies, including Kymab, BiomX, LEO Pharma, GlaxoSmithKline, Arjil Pharmaceuticals, SCM Lifescience, Sun Pharmaceutical Industries Limited, Brickell Biotech Inc, Dermira, AstraZeneca, Kyowa Kirin, UCB Biopharma, Arcutis Biotherapeutics, and others. Kymab, BiomX, LEO Pharma, GlaxoSmithKline, Arjil Pharmaceuticals, SCM Lifescience, Sun Pharmaceutical Industries Limited, Brickell Biotech Inc, AstraZeneca, Kyowa Kirin, UCB Biopharma, Arcutis Biotherapeutics, Vanda Pharmaceuticals, Kyowa Kirin, Sanofi, KeyMed Biosciences, Asana BioSciences, Bristol-Myers Squibb, RAPT Therapeutics, Allakos, Novartis, BioMimetix, Shanghai Hengrui Pharmaceutical Co, Connect Biopharma, Pfizer, Evommune, Inc., Fresh Tracks Therapeutics, Biosion, Chia Tai Tianqing Pharmaceutical, Reistone Biopharma Company Limited, JW Pharmaceutical, Oneness Biotech, Alphyn Biologics, selectION, UNION Therapeutics, Ichnos Scien, among others.
DelveInsight’s Pharma Competitive Intelligence Service: Through its CI solutions, DelveInsight provides its clients with real-time and actionable intelligence on their competitors and markets of interest to keep them stay ahead of the competition by providing insights into the latest therapeutic area-specific/indication-specific market trends, in emerging drugs, and competitive strategies. These services are tailored to the specific needs of each client and are delivered through a combination of reports, dashboards, and interactive presentations, enabling clients to make informed decisions, mitigate risks, and identify opportunities for growth and expansion.
Other Business Pharmaceutical Consulting Services
Healthcare Conference Coverage
Discover how a mid-pharma client gained a level of confidence in their soon-to-be partner for manufacturing their therapeutics by downloading our Due Diligence Case Study
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences.
Connect with us at LinkedIn

Contact Us Shruti Thakur info@delveinsight.com +14699457679 www.delveinsight.com
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
